Background
Despite an improvement in treatment response, high-risk multiple myeloma (MM) patients (pts) experience early relapse and short disease-free survival. Together with more validated high-risk features, high levels of circulating plasma cells (high CPC) have been considered a marker of aggressive disease and poor outcome (F. Gay et al, ASH 2019; W.I. Gonsalves et al, Am J Hematol 2020). To date, there are no uniform data on the optimal cut-off predictive of clinical outcome. No prospective data on CPC are available in the setting of novel-drug clinical trials with comprehensive baseline evaluation and minimal residual disease (MRD) assessment.
Aims
1) To identify the best cut-off for CPC to predict progression-free survival (PFS);
2) to assess the impact of high CPC levels on the clinical outcome of newly diagnosed (ND)MM pts in the context of concomitant risk features and MRD evaluation.
Methods
In the multicenter randomized FORTE clinical trial, 474 NDMM pts ≤65 years were randomized (R1) to receive either: carfilzomib-lenalidomide-dexamethasone (KRd) induction-autologous stem-cell transplant-KRd consolidation (KRd_ASCT); KRd for 12 cycles (KRd12); or carfilzomib-cyclophosphamide-dexamethasone (KCd) induction-ASCT-KCd consolidation (KCd_ASCT). Thereafter, pts were randomized (R2) to maintenance treatment with lenalidomide alone (R) or plus carfilzomib (KR). MRD was assessed by 2nd-generation multiparameter flow cytometry (MFC, sensitivity 10-5) in pts who achieved ≥very good partial response before maintenance and then every 6 months.
At diagnosis, single-platform FC was used to sort and count CPC. Receiver Operating Characteristic (ROC) analysis was used to define a cut-off based on PFS at 36 months as outcome. Correlations between high CPC and the most important baseline prognostic features (age, International Staging System (ISS), lactate dehydrogenase (LDH), chromosomal abnormalities (CA) by FISH [(del17p, t(4;14), t(14;16), t(11;14), amp1q, del1p, del13], Revised-ISS (R-ISS)) were explored. Hence, we performed a multivariate (MV) analysis to assess the impact of high CPC on the achievement of MRD negativity, on PFS and OS. Finally, we evaluated the impact of baseline CPC and MRD achievement.
Results
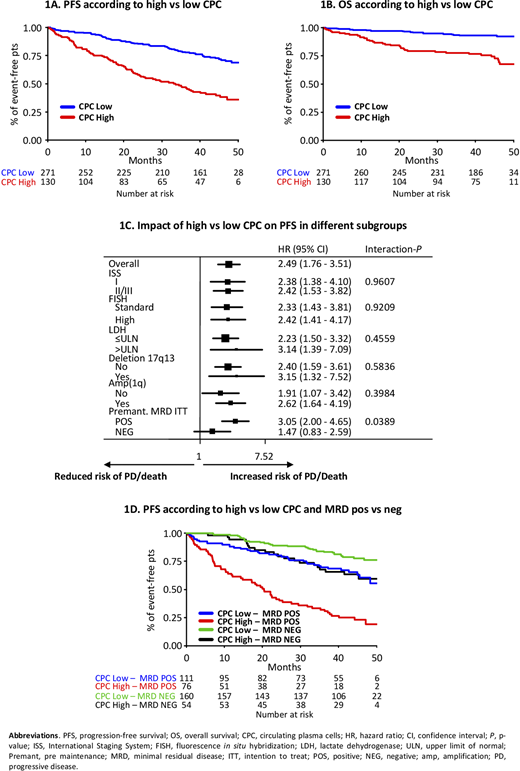
CPC analysis was performed in 401/474 pts at diagnosis; median follow-up was 44.2 months (39.6-47.9) and baseline features were similar to those reported in the overall FORTE population. Median CPC were 0.02% (IQR 0-0.14). The optimal CPC cut-off to predict PFS (ROC analysis) was 0.07% (5 cells/ul, 0.005 x109/l) and was consistent with a cut-off previously identified as a predictor of sustained MRD negativity (MRDsus12; L. Bertamini et al, EHA 2020). High-CPC pts (>0.07%) were 130/401 (32%), while 271/401 (68%) had low CPC (≤0.07%). The proportion of high-CPC pts was comparable among treatment arms.
Baseline features significantly associated with high CPC in a MV analysis were: ISS II/III, high LDH, amp1q, t(4;14), t(14;16) and bone marrow plasma cells (>60%).
Regarding PFS, in a MV analysis adjusted for R-ISS and R1 treatment, including all the baseline features, high CPC were associated with a lower PFS (HR 2.49, 95% CI 1.76-3.51, P<0.0001; 3-year PFS rates 47% for high CPC vs 78% for low CPC; Fig. 1A). Similarly, patients with high CPC had a worse OS compared with patients with low CPC (3-year OS rates 78% for high CPC vs 93% for low CPC; HR 2.85, 95% CI 1.56-5.19, P=0.0006; Fig. 1B).
The impact of baseline CPC levels on PFS was consistent in all high-risk subgroups (Fig. 1C), except in those patients who achieved pre-maintenance MRD negativity [(neg); interaction P=0.03]. Low-CPC and MRD-neg pts showed the best outcome with a 3-year PFS of 84%. Low-CPC MRD-positive (pos) and high-CPC MRD-neg pts had similar 3-year PFS (70% vs 68%). High-CPC MRD-pos pts had a dismal outcome (3-year PFS 32%; Fig. 1D).
Conclusion
High CPC with a cut-off of 0.07% (5 cells/ul, 0.005 x109/l) is a strong and independent high-risk factor, predicting a shorter PFS and OS even in the context of other high-risk features. The achievement of MRD neg independently improved the poor prognosis of high-CPC patients.
D'Agostino:GSK: Membership on an entity's Board of Directors or advisory committees. Galieni:Janssen: Honoraria; AbbVie: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Molica:Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Tacchetti:Oncopeptides: Honoraria; AbbVie: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Musto:Amgen: Honoraria; Celgene: Honoraria. Gay:GSK: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boccadoro:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Mundipharma: Research Funding. Oliva:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees.
The presentation includes discussion of off-label use of a drug or drugs for the treatment of multiple myeloma (including carfilzomib, cyclophosphamide, lenalidomide and dexamethasone).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal